
By BIOTUESDAYS
PharmaCyte Biotech (OTCQB:PMCB) is developing cellular therapies for its lead pancreatic cancer program and for diabetes that are based on a proprietary cellulose-based live cell encapsulation technology known as a Cell-in-a-Box.
“When used for pancreatic cancer treatment, the combination of Cell-in-a-Box encapsulation plus a cancer prodrug, which needs to be converted to its cancer-killing form by the cells in the Cell-in-a-Box capsules, turns out to be a completely unique approach to the treatment of pancreatic cancer,” CEO, president and general counsel, Kenneth L. Waggoner, says in an interview with BioTuesdays.
“In very early clinical trials, this combination produced a strong antitumor effect at the tumor site, with little-to-no treatment-related side effects,” he adds.
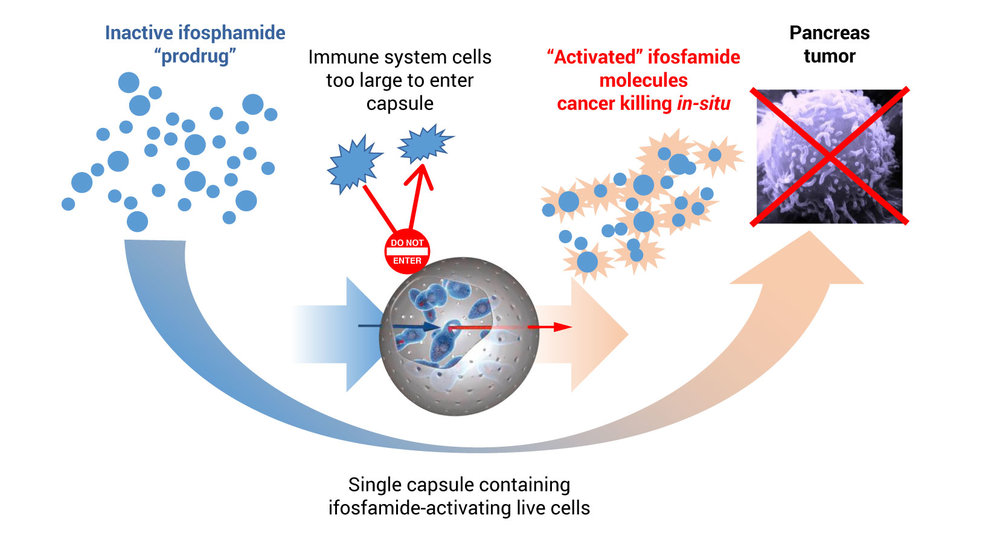
Mr. Waggoner explains that to treat pancreatic cancer, PharmaCyte’s platform technology encapsulates genetically modified live cells that are used with a cancer prodrug, ifosfamide. These modified encapsulated cells are implanted in the blood supply as close as possible to the tumor site.
After implantation, ifosfamide is given intravenously at one-third the normal dose, he points out. When the IV ifosfamide reaches the encapsulated cells, it is designed to flow through pores in the capsules, where the genetically modified live cells produce an enzyme that converts ifosfamide into its cancer-killing form, he adds.
Mechanism of Action
Mr. Waggoner contends that the live cells inside the capsule act as a “bio-artificial liver” activating the chemotherapy at the site of the cancer.
“We use the term bio-artificial liver because if the Cell-in-a-Box capsules were not present near the tumor, the inactive prodrug would not be converted into its active form until it passed through the liver,” he says. “Thereafter, the activated ifosfamide, which has a short biological half-life, would be very diluted by the time it reached the pancreatic tumor and the anticancer effectiveness of the ifosfamide would be quite low.”
Mr. Waggoner contends that the implanted capsules do not impede the blood supply to the pancreas and thus to the tumor. This “targeted chemotherapy has proven effective and safe to use in earlier clinical trials, with little to no treatment-related side effects.”
A single implantation consists of 300 Cell-in-a-Box capsules, which are each the size of a head of a pin, Mr. Waggoner says, with each capsule containing approximately 20,000 genetically modified live cells.
Mr. Waggoner points out that a normal dose of ifosfamide has shown success in treating some cancers, including pancreatic cancer, but clinicians are reluctant to use a normal dose because of its severe toxicity.
“If this technology can be solidly proven in the clinic, it will change how certain types of solid tumors are treated for a very long time,” he contends.
 Gerald W. Crabtree, COO of PharmaCyte, says the Cell-in-a-Box capsules, which are made of bio-inert cellulose (cotton), have been shown to be safe, effective and durable for at least two years in the body. In addition, the pores in the capsules are too small for immune system cells to enter or encapsulated live cells to leave.”
Gerald W. Crabtree, COO of PharmaCyte, says the Cell-in-a-Box capsules, which are made of bio-inert cellulose (cotton), have been shown to be safe, effective and durable for at least two years in the body. In addition, the pores in the capsules are too small for immune system cells to enter or encapsulated live cells to leave.”
Dr. Crabtree explains that other live cell encapsulation technologies have used alginate, which is derived from seaweed, and alginate derivatives, but many of these tend to produce more of a gel than a capsule. “In addition, alginate-based capsules tend to be far less robust and less stable than our Cell-in-a-Box capsules, and they can’t be frozen for long-term storage or the long-distance shipment.”
Pancreatic cancer is the third leading cause of cancer-related deaths in the western world, with an overall survival rate of 8%. Some 72% of patients will die within the first year of diagnosis and more than 90% will die within two years after diagnosis. Patients have less than six-months average life expectancy after diagnosis without treatment.
In addition, pancreatic cancer is usually not diagnosed until it is advanced and inoperable. However, advances in imaging technologies have enabled the disease to be diagnosed earlier. Still, there is no cure unless the tumor is surgically removed in its earliest stages.
Since the first chemotherapy drug, gemcitabine, was approved by the FDA for pancreatic cancer in 1996, some 40 unsuccessful pivotal trials have been conducted to find a combination therapy to improve on gemcitabine alone, Dr. Crabtree recalls
The current standard of care is a combination of gemcitabine and Abraxane. The FDA approved the combination chemotherapy in 2013. While there are severe side effects associated with gemcitabine plus Abraxane therapy, the combination has increased the percentage of one-year survivors to 38% from 22% with gemcitabine alone.
FOLFIRINOX, a combination of four chemotherapy agents – folinic acid, 5-fluorouracil, irinotecan and oxaliplatin – has been used outside of North America for patients with metastatic and locally advanced pancreatic cancer, and is now used in America in some cases, before surgery to shrink the tumor as much as possible. However, FOLFIRINOX does not have marketing approval and comes with severe side effects.
In the 1990s, a Danish company, Bavarian Nordic, sponsored two small clinical trials in Europe in patients with stage 3 and 4 pancreatic cancer. Although historical data were used as a control in these trials, it was possible to compare the data from patients that had received gemcitabine alone, Dr. Crabtree recalls.
In these trials, an earlier version of the Cell-in-a-Box plus low doses of ifosfamide was used. The Cell-in-a-Box-based therapy was found to be safe, effective and well tolerated by patients. Mr. Waggoner says that in some cases, a patient’s tumor went from inoperable to operable.
PharmaCyte, which licensed the Cell-in-a-Box technology in 2013, is currently finalizing an IND that, if approved by the FDA, would allow PharmaCyte to conduct a Phase 2b trial in the U.S. The trial would be scheduled to start in the first quarter of 2020.
Mr. Waggoner says the company is targeting a critical unmet medical need that exists for patients with advanced pancreatic cancer whose tumors are locally advanced, non-metastatic and inoperable, and no longer respond to Abraxane plus gemcitabine or FOLFIRINOX therapy.
“The goal of the trial is to show that PharmaCyte’s therapy for pancreatic cancer can serve as a consolidation therapy with Abraxane plus gemcitabine or FOLFIRINOX, where no satisfactory consolidation therapy currently exists,” he adds.
The company plans to randomize 100 patients into three groups. Fifty patients will receive PharmaCyte’s pancreatic cancer therapy, while the other 50 will receive capecitabine, a chemotherapy, plus external beam radiation, or stereotactic body radiation alone.
Mr. Waggoner says each patient in the PharmaCyte therapy group will receive a single implantation of 300 Cell-in-a-Box capsules, which will be implanted in the patients’ bodies three days before they begin receiving multiple courses of low-dose ifosfamide. The administration of the ifosfamide will continue until patients become refractory to the drug or their disease progresses.
The primary endpoint of the trial is progression-free survival. Among secondary endpoints are overall survival; objective response rate, complete and partial responses; and determining the number of patients whose tumors are converted from an inoperable state to an operable one post-treatment, he adds.
Three internationally renowned pancreatic cancer oncologists – Dr. Daniel Von Hoff, Dr. Manuel Hidalgo and Dr. Matthias Löhr – played leading roles in identifying and designing the Phase 2b trial.
Dr. Von Hoff has been a leading figure in the development of cancer drugs for many years; and Dr. Löhr was the principal investigator (PI) of the earlier Phase 1/2 trials and is currently chairman of PharmaCyte’s medical and scientific advisory board. Dr. Hidalgo is the PI for the company’s upcoming Phase 2b study.
PharmaCyte has partnered with Austrianova Singapore Pte. to manufacture the Cell-in-a-Box clinical trial material at its cGMP manufacturing facility in Bangkok, Thailand. “Production is being validated on an ongoing basis, and we expect release of clinical trial material in the U.S. during the fourth quarter of 2019,” Mr. Waggoner offers.
PharmaCyte has orphan drug designation for its pancreatic cancer therapy in the U.S. and Europe. It also has eligibility under the Biologics Price Competition and Innovation Act, which provides 12 years of market exclusivity in the U.S. and Europe.
PharmaCyte’s patent portfolio includes the encapsulation process, which uses unique patent-protected cellulose sulphate and is a pivotal ingredient in the formation of the Cell-in-a-Box capsules; patents protecting genetically engineered cells that convert a cancer prodrug into its active form; as well as Melligen insulin-producing cells that may be a pivotal part of the company’s diabetes program.
The company’s therapy for Type 1 diabetes and insulin-dependent Type 2 diabetes, though still in the preclinical stage, involves encapsulating genetically modified liver cells that are called Melligen cells. PharmaCyte also will be examining the use of stem cells and beta islet cells that have been encapsulated using the Cell-in-a-Box technology and then implanting these capsules in the body.
Mr. Waggoner explains that Melligen cells are human liver cells that have been modified to produce, store and release insulin in response to concentrations of glucose in the body. In addition, Melligen cells have demonstrated the ability to reverse the diabetic condition in immunosuppressed diabetic mice.
PharmaCyte has an exclusive worldwide license to use Melligen insulin-producing cells to treat diabetes.
“In a way that is analogous to how Cell-in-a-Box capsules protect the cells from immune system attack in the body when they are used as part of a pancreatic cancer treatment, they should do the same thing for insulin-producing cells,” Mr. Waggoner says. “These types of cells are designed to function as a bio-artificial pancreas for purposes of insulin production.”
Pipeline
• • • • •
To connect with PharmaCyte, or any of the other companies featured on BioTuesdays, send us an email at [email protected].
